SimplusDx
Simplified Testing. Powerful Insights.
About Us
Founded in 2024 as a spinout from Duke University, SimplusDx is dedicated to making point-of-care testing a standard in clinical diagnostics. Our mission is to democratize access to laboratory-grade testing by delivering simple, easy-to-use tests directly to healthcare providers and patients, wherever they are. Our innovative solutions simplify and enhance diagnostic workflows, transforming complex tests into actionable insights. With precise, on-the-spot results, we empower patients and healthcare providers to make immediate, informed decisions that improve health outcomes.
Founding Team

Jake Heggestad, PhD
Chief Executive Officer

David Kinnamon, PhD
Chief Technology Officer

Cassio Fontes, PhD
Chief Scientific Officer
Board of Directors

Ashutosh Chilkoti, PhD
Co-founder, Chairman

Angus Hucknall, PhD
Co-founder, Director
Our Technology
We set out to develop a point-of-care immunoassay platform that can be performed in decentralized locations, while matching the performance of centralized laboratory-based tests. The D4 assay fulfills this goal by delivering rapid, reliable results independent of clinical testing infrastructure. Its unique ‘non-fouling’ synthetic polymer surface minimizes noise from complex samples like whole blood, enabling highly sensitive, accurate, and precise performance.
The D4 Assay



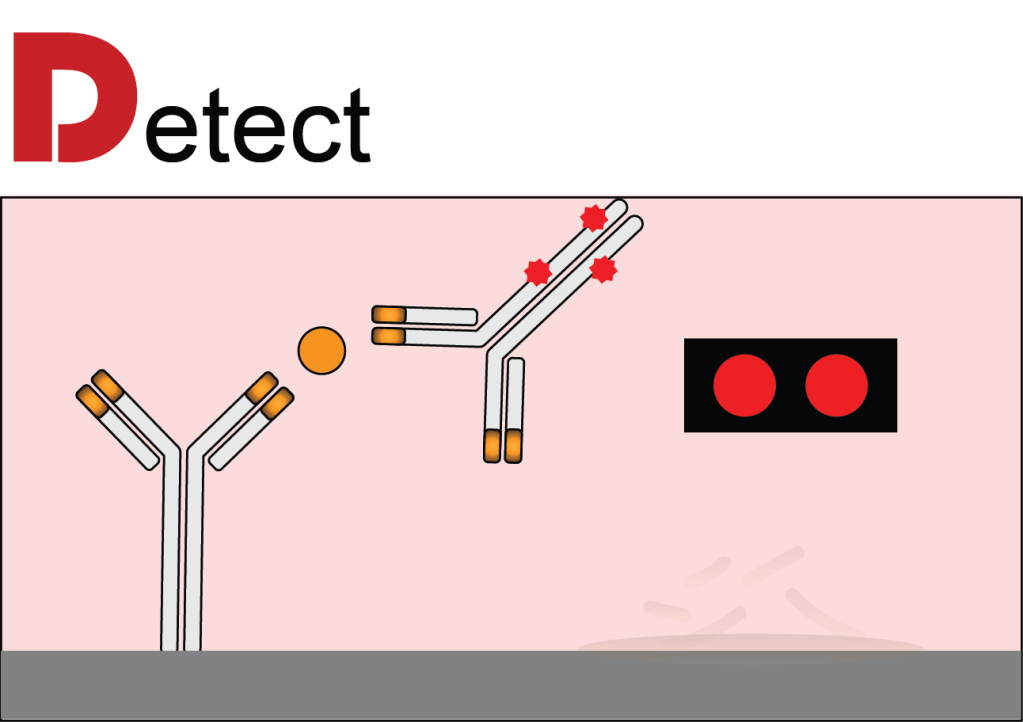
A drop of blood is Dispensed onto the chip, initiating the Dissolution of a fluorescently labeled detection reagent. Following this, the reagent Diffuses and binds to form sandwich complexes with the capture reagent, which are then Detected to quantify the analyte of interest. All of these processes are carried out within a user-friendly cartridge and our handheld analyzer offers a portable solution for obtaining quick, accurate results right at the point-of-care with the push of a button. We are using these assays for a variety of applications, including therapeutic drug monitoring, management of chronic conditions, acute disease screening, and more!
Advantages of our approach

High sensitivity
Detect clinically relevant biomarkers at picomolar concentrations directly from complex samples (e.g., whole blood)

Quantitative readout
Receive quantitative results in seconds, rather than traditional binary readouts from other POCTs

Multiplex compatible
Test for a variety of biomolecules (e.g., proteins, antibodies, small molecules) with a modular and multiplexable test format

Short turnaround time
Assays can be conducted within 30-minutes, giving patients and healthcare providers access to actionable information quickly

User-friendly operation
Automated results with few hands on requirements, easily integrating into clinical workflows and / at home patient testing

Accessible and inexpensive
Analyzer and consumables are shelf-stable and low cost, making it feasible to test in decentralized locations (e.g., at home, clinics)
Scientific Publications

Inkjet-printed point-of-care immunoassay on a nanoscale polymer brush enables subpicomolar detection of analytes in blood

COVID-19 Diagnosis and SARS-CoV-2 Strain Identification by a Rapid, Multiplexed, Point-of-Care Antibody Microarray

Environmentally Resilient Microfluidic Point-of-Care Immunoassay Enables Rapid Diagnosis of Talaromycosis
Trusted by



News

October 8th, 2025
SimplusDx joining the VentureWell Aspire MedTech Fall 2025 Cohort
SimplusDx was selected as one of 16 teams for the Fall 2025 Aspire Medtech investor-engagement program. The program provides intensive training, investor mentoring, and support to help medtech startups advance fundraising and prepare for due diligence. Read more here.

September 22nd, 2025
SimplusDx selected as a semi-finalist to the NC IDEA SEED grant
We are proud to share that SimplusDx, Inc. was selected as a semi-finalist for the NC IDEA SEED Fall 2025 grant. SimplusDx was among 20 companies selected from a pool of over 175 applications. Read more here.

July 1st, 2025
SimplusDx awarded NIH grant to develop a rapid test for tacrolimus monitoring
SimplusDx has been awarded a highly competitive SBIR grant through the National Institute of Health (NIH) for the development of a rapid, point-of-care test for tacrolimus detection.






